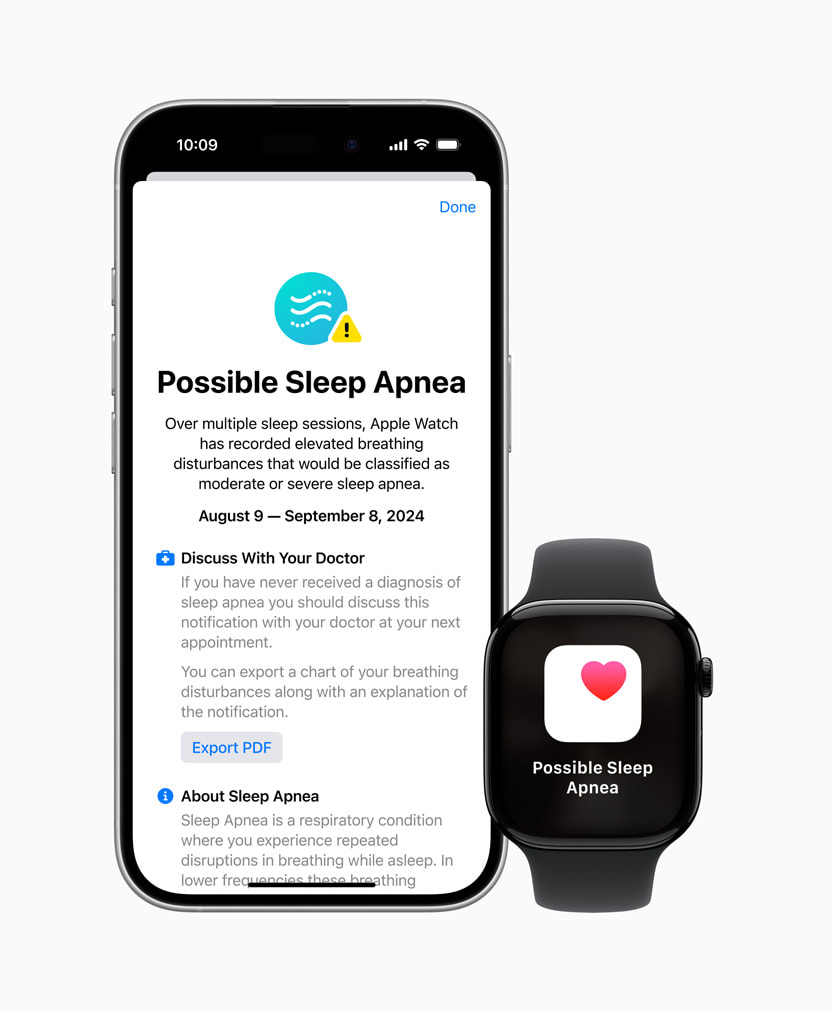
The US Food and Drug Administration (FDA) on Monday published approval for the ‘sleep apnea notification’ feature on the Apple Watch Series 9, Series 10, and Watch Ultra 2.
The FDA categorizes the feature as an “over-the-counter device to assess the risk of sleep apnea.” Apple emphasizes that this addition is not a diagnostic tool. Instead, it will encourage users to seek a formal diagnosis from a healthcare provider, reports TechCrunch.
Also Read: Apple AirPods Pro gets FDA approval to serve as hearing aids
Sleep apnea is a condition that leads to shallow breathing or repeated pauses in breathing during sleep and is linked to various symptoms. The Mayo Clinic indicates that it can result in insomnia, headaches, daytime sleepiness, and other long-term conditions.
The feature was announced at Apple’s ‘It’s Glowtime’ event, where the tech giant revealed the iPhone 16 lineup and other products. The feature will arrive as part of the imminent watchOS 11 release. After activation, it needs 10 nights of sleep tracking data within a 30-day period to assess if a user might have the condition. It also provides insights into nightly sleep disturbances using the built-in accelerometer.
Also Read: Apple debuts AI-powered iPhone 16 series: Check India pricing, availability
Apple Watch Series 10 starts at Rs 46,900, while Apple Watch Ultra 2 starts at Rs 89,900, and comes available in natural and black titanium. The smartwatches will be available for purchase starting September 20.
Meanwhile, Apple’s AirPods Pro has also received FDA approval to serve as “hearing aids”. The FDA has authorised the first over-the-counter hearing aid software device, Hearing Aid Feature (HAF), intended to be used with compatible versions of the Apple AirPods Pro headphones.